Table of Contents
Matter is composed of tiny particles called atoms and molecules. These building blocks are too small to be seen with the naked eye. Matter exists in different forms on Earth, including solids, liquids, and gases. In a solid, the particles are attracted to each other and vibrate in position. Liquids have particles that are close together and able to slide past one another. Gases have particles that are far apart and constantly moving. Atoms and molecules form the foundation of these various states of matter.
The Structure of Atoms
Atoms are the smallest particles of an element that have the same chemical properties. They are the building blocks of matter and consist of three subatomic particles: protons, neutrons, and electrons.
Protons and neutrons are located in the nucleus, the central part of the atom. Protons carry a positive charge, while neutrons have no charge. These particles are relatively massive compared to electrons.
Electrons, on the other hand, are negatively charged and are found outside the nucleus in electron shells or orbits. They are much smaller and lighter than protons and neutrons.
The structure of an atom can be summarized as follows:
The nucleus contains protons and neutrons.
Electrons are found outside the nucleus in electron shells.
The exact number of protons, neutrons, and electrons in an atom determines its atomic number, atomic mass, and chemical properties. Atoms can have different numbers of these subatomic particles, resulting in varying elements and isotopes.
Here’s a table showcasing the properties of protons, neutrons, and electrons:
| Particle | Charge | Relative Mass | Location |
|---|---|---|---|
| Protons | Positive | 1 | In the nucleus |
| Neutrons | No charge | 1 | In the nucleus |
| Electrons | Negative | ~0.0005 | Outside the nucleus |
Understanding the structure of atoms is crucial for comprehending the behavior and interactions of matter at the atomic level. This knowledge forms the foundation of various scientific disciplines, including chemistry and physics.
The Evolution of Atomic Models
The study of atomic models has evolved over the years, leading to significant scientific breakthroughs in understanding the structure of atoms. This section explores the contributions of J.J. Thomson, Ernest Rutherford, and Niels Bohr in shaping our understanding of the atomic realm.
J.J. Thomson and the Plum Pudding Model
J.J. Thomson made a groundbreaking discovery when he identified the existence of electrons. He proposed a model known as the plum pudding model, where electrons are embedded in a positive sphere. In this model, the atom is envisioned as a uniform distribution of positive charge with negatively charged electrons distributed throughout.
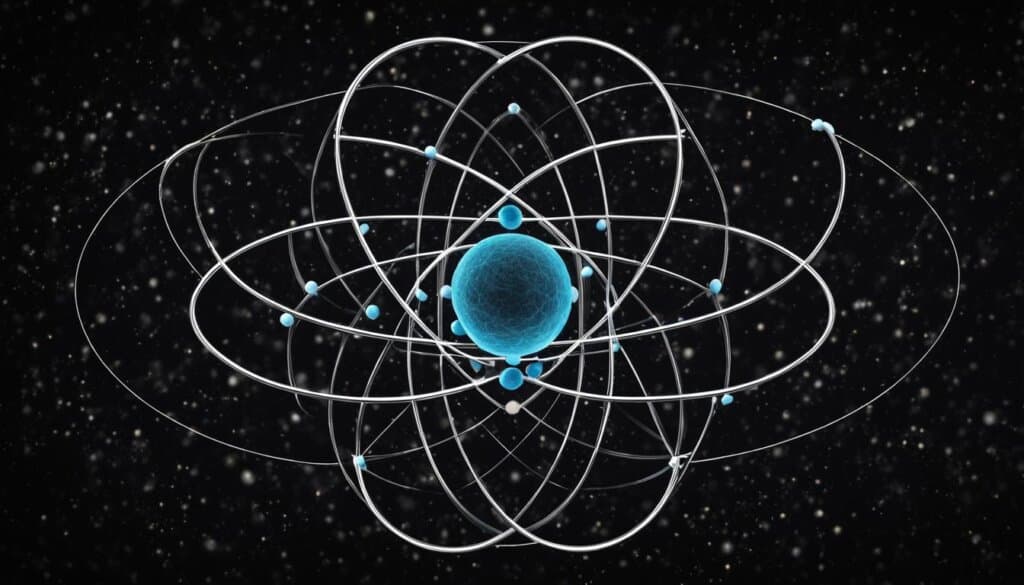
Ernest Rutherford and the Nuclear Model
Ernest Rutherford conducted the famous gold foil experiment, which revolutionized our understanding of atomic structure. His observations led to the proposal of the nuclear model, which suggests that an atom consists of a small, dense nucleus at its center, containing protons and neutrons. The electrons, with their negative charge, orbit around the nucleus.
Niels Bohr and Electron Orbits
Building upon Rutherford’s model, Niels Bohr introduced the concept of electron orbits. He proposed that electrons exist in fixed energy levels or orbits outside the nucleus. The electron transitions between these orbits result in the emission or absorption of energy, a fundamental process in the study of atomic behavior.
“The atom is not simply a particle, but a dynamic system with electrons occupying discrete energy levels.” – Niels Bohr
This new understanding of electron orbits provided a basis for explaining the spectral lines observed in elements and helped further refine our understanding of atomic structure.
| Scientist | Contribution |
|---|---|
| J.J. Thomson | Discovered the electron and proposed the plum pudding model |
| Ernest Rutherford | Conducted the gold foil experiment and proposed the nuclear model |
| Niels Bohr | Expanded on Rutherford’s model and introduced the concept of electron orbits |
Isotopes and Atomic Weight
Isotopes are different versions of atoms with the same number of protons but varying numbers of neutrons. This means that isotopes of an element have different atomic weights. For example, carbon, which is commonly found in nature, has two stable isotopes: carbon-12 and carbon-13. Carbon-12 has 6 protons and 6 neutrons, while carbon-13 has 6 protons and 7 neutrons. These isotopes have slightly different atomic weights due to the extra neutron in carbon-13.
The average atomic weight of an element is determined by the relative abundance of each isotope. Abundance refers to the proportion of one isotope compared to the total number of isotopes of that element. For instance, carbon-12 is the most abundant isotope of carbon, making up about 98.9% of naturally occurring carbon, while carbon-13 accounts for the remaining 1.1%. The average atomic weight of carbon is then calculated by multiplying the atomic weight of each isotope by its abundance and summing these values.
It’s important to note that the average atomic weights shown on the periodic table have been adjusted to reflect the abundance of each isotope. This is because the presence of isotopes can affect the average atomic weight, making it deviate from the total number of nucleons in the atom’s nucleus. Therefore, the value displayed on the periodic table provides a more accurate representation of the atomic weight of an element based on its naturally occurring isotopes.
FAQ
What is matter?
Matter is made up of tiny particles called atoms and molecules. These particles are too small to be seen with the naked eye. Matter on Earth exists in the form of solid, liquid, or gas.
What are atoms?
Atoms are the smallest particles of an element that have the same chemical properties. They consist of three subatomic particles: protons, neutrons, and electrons. Protons and neutrons are located in the nucleus, which is the central part of the atom. Electrons are negatively charged and are found outside the nucleus in electron shells.
Who discovered the electron?
J.J. Thomson discovered the electron and proposed the plum pudding model, where electrons are embedded in a positive sphere.
What is the nuclear model of the atom?
The nuclear model of the atom was proposed by Ernest Rutherford. In this model, the atom has a small, dense nucleus and electrons orbit around it.
How did Niels Bohr contribute to the atomic model?
Niels Bohr expanded on Rutherford’s model and proposed that electrons exist in fixed energy levels or orbits outside the nucleus.
What are isotopes?
Isotopes are varieties of atoms with the same number of protons but different numbers of neutrons. Elements can have multiple isotopes with different atomic weights.
How does the presence of isotopes affect atomic weight?
The average atomic weight of an element is determined by the abundance of each isotope. The values shown on the periodic table are the average atomic weights, adjusted for the abundance of each isotope.